Difference between revisions of "Chapter 6"
(→6.6.3 Special Handling Methods) |
(→6.6.3 Special Handling Methods) |
||
| Line 243: | Line 243: | ||
'''6.6.3.5 Encapsulation and Layering''' | '''6.6.3.5 Encapsulation and Layering''' | ||
| − | Encapsulation and layering involves placing acid producing and acid consuming materials (typically waste rock) in geometries designed to control or limit ARD | + | Encapsulation and layering involves placing acid producing and acid consuming materials (typically waste rock) in geometries designed to control or limit ARD. The effectiveness of the encapsulation and layering is governed by availability of materials, the general balance between acid producing and acid neutralizing materials, the type and reactivity of acid-consuming material, deposit geometry, the nature and flow of water through the deposit, and chemical armoring of alkaline materials (MEND, 1998a and 2001; Miller et al., 2003 and 2006). Layering of waste rock and tailings materials is discussed below in Section 6.6.3.7. |
<div id="Figure 6-6" style="text-align:center">'''Figure 6-6: Example Waste Rock Encapsulation Strategy'''<br /> | <div id="Figure 6-6" style="text-align:center">'''Figure 6-6: Example Waste Rock Encapsulation Strategy'''<br /> | ||
| Line 250: | Line 250: | ||
'''6.6.3.6 Blending''' | '''6.6.3.6 Blending''' | ||
| − | Blending is the mixing of waste rock types of varying acid generation and neutralization potential to create a deposit that generates a discharge of acceptable quality. | + | Blending is the mixing of waste rock types of varying acid generation and neutralization potential to create a deposit that generates a discharge of acceptable quality. The effectiveness of blending as an option depends on the availability of materials and the mine plan, the general stoichiometric balance between acid producing and acid neutralizing materials, geochemical properties, reactivity of waste rock types, flow pathways created within the deposit, and the extent of mixing and method of blending. Homogeneous and thorough mixing is generally required to achieve maximum benefit (MEND, 1998a and 2001). Evidence from field trials indicates limited success with mixing of waste rock types using haul trucks, but better mixing of waste rock with limestone was achieved using a conveyor system and stacker (Miller et al., 2006). |
| − | + | Operational experience indicates that, for effective blending of PAG rock with limestone, it is essential that all size fractions within the blend be at least acid-base neutral (i.e., NPR (ANC/MPA) of at least 1). Since the run of mine particle size of limestone is generally coarser than PAG rock, the acid base balance of the bulk waste rock needs to be greater than balanced. Experience with rock types at Freeport (Indonesia) and Ok Tedi (Papua New Guinea) indicate that well mixed PAG and limestone rock needs to have a bulk NNP of more than 150 kg CaCO<sub>3</sub>/t (or NAPP less than 150 kg H<sub>2</sub>SO<sub>4</sub>/t). The actual blend will depend on site factors and particle size distribution for each rock type. | |
| − | '''6.6.3.7 | + | '''6.6.3.7 Co-disposal''' |
| − | + | Co-disposal is the disposal of waste rock with tailings. Co-disposal can take several forms, which vary depending on the degree of mixing, as summarized in Table 6-1 (Wickland et al, 2006). | |
| − | <div id="Table 6-1" style="text-align:center">'''Table 6-1: Forms of | + | <div id="Table 6-1" style="text-align:center">'''Table 6-1: Forms of Co-disposal (from Wickland et al., 2006)'''</div> |
<table align="center" border="1" cellspacing="0" cellpadding="4" width="576"> | <table align="center" border="1" cellspacing="0" cellpadding="4" width="576"> | ||
<tr> | <tr> | ||
| − | <td colspan="2" align="center" valign="top" bgcolor="#dddddd"><strong> | + | <td colspan="2" align="center" valign="top" bgcolor="#dddddd"><strong>Co-disposal Type</strong></td> |
</tr> | </tr> | ||
<tr> | <tr> | ||
| Line 269: | Line 269: | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| − | <td width="444" valign="top">Pumped | + | <td width="444" valign="top">Pumped co-disposal – Coarse and fine materials are pumped to impoundments for disposal (segregation occurs on deposition).</td> |
<td width="132" rowspan="5" align="center" valign="top">[[Image:Fatuparrow.gif]]</td> | <td width="132" rowspan="5" align="center" valign="top">[[Image:Fatuparrow.gif]]</td> | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| − | <td width="444" valign="top">Layered | + | <td width="444" valign="top">Layered co-mingling –Layers of waste rock and tailings are alternated.</td> |
</tr> | </tr> | ||
<tr> | <tr> | ||
| Line 288: | Line 288: | ||
| − | + | Co-disposal has the potential to limit ARD as follows: | |
| − | * | + | *Co-disposal of highly reactive waste rock within saturated tailings impoundments |
| − | *Layered | + | *Layered co-mingling of thickened tailings and waste rock |
*Thorough mixing of paste or filtered tailings with waste rock to create “paste rock” | *Thorough mixing of paste or filtered tailings with waste rock to create “paste rock” | ||
| − | For ideal mixing, the void space of waste rock particles are filled with finer tailings particles, thereby limiting the flow of oxygen and water relative to deposits of waste rock alone. Amendment of tailings with alkaline materials is possible, and | + | For ideal mixing, the void space of waste rock particles are filled with finer tailings particles which typically have a higher moisture content, thereby limiting the flow of oxygen and water relative to deposits of waste rock alone. Amendment of tailings with alkaline materials is possible, and co-disposed materials will have a lower rate of ARD production than only blending of waste rock types. Benefits and considerations for co-disposal are listed in Table 6-2. |
| − | <div id="Table 6-2" style="text-align:center">'''Table 6-2: Benefits and Considerations of | + | <div id="Table 6-2" style="text-align:center">'''Table 6-2: Benefits and Considerations of Co-disposal'''</div> |
<table align="center" border="1" cellspacing="0" cellpadding="4"> | <table align="center" border="1" cellspacing="0" cellpadding="4"> | ||
| Line 326: | Line 326: | ||
</table> | </table> | ||
| − | Pumped | + | Pumped co-disposal of coarse and fine coal beneficiation wastes has been implemented at coal mines in the United States of America, Australia, and Indonesia since the 1990s (Williams et al., 1995), though pumped co-disposal typically results in a segregated deposit. Co-disposal is used in South African coal operations where coal slurry is placed within a dam constructed of coarse reject. However, mixing waste rock and tailings for blended co-disposal and paste rock is in the research stage, and has not been implemented at full scale at operating hard rock mines. |
'''6.6.3.8 Permafrost and Freezing''' | '''6.6.3.8 Permafrost and Freezing''' | ||
| Line 332: | Line 332: | ||
Permafrost or ice covers approximately 25% of the earth’s surface, and the mine operator may take advantage of cold conditions to control weathering and drainage. Permafrost is generally defined as ground that remains below 0º Celsius for more than 2 years. The term permafrost does not imply ice or water content. | Permafrost or ice covers approximately 25% of the earth’s surface, and the mine operator may take advantage of cold conditions to control weathering and drainage. Permafrost is generally defined as ground that remains below 0º Celsius for more than 2 years. The term permafrost does not imply ice or water content. | ||
| − | Freezing of materials to control acid generation has been used as a strategy to control ARD at several cold sites (MEND, | + | Freezing of materials to control acid generation has been used as a strategy to control ARD at several cold sites (MEND, 2004b). Chemical activity does not stop at 0º Celsius, and freezing point depression because of chemical content of pore water may result in unfrozen water present in mine waste materials at temperatures well below freezing. |
| − | Periodic warming of the surface during summer periods in zones of permafrost results in thaw within an upper “active zone.” Strategies for limiting chemical weathering may include a cover that prevents penetration of the active zone into PAG materials so that the PAG materials remain frozen. | + | Periodic warming of the surface during summer periods in zones of permafrost results in thaw within an upper “active zone.” Strategies for limiting chemical weathering may include a cover that prevents penetration of the active zone into PAG materials so that the PAG materials remain frozen (MEND, 2009). |
Tailings deposition planning may be optimized to promote freezing during winter months and limit thawing during summer months. Thermal analysis is required to predict long-term freezing of reactive materials. Experience has shown that tailings will freeze on cold sites, such as at the Nanisivik Mine, Nunavut, Canada (Claypool et al., 2007). | Tailings deposition planning may be optimized to promote freezing during winter months and limit thawing during summer months. Thermal analysis is required to predict long-term freezing of reactive materials. Experience has shown that tailings will freeze on cold sites, such as at the Nanisivik Mine, Nunavut, Canada (Claypool et al., 2007). | ||
| − | As a general rule, permafrost encapsulation requires approximately -8º Celsius mean annual air temperature. One possible limitation of relying on freezing alone is the potential for climate change associated with global warming (MEND, 2001 and | + | As a general rule, permafrost encapsulation requires approximately -8º Celsius mean annual air temperature. One possible limitation of relying on freezing alone is the potential for climate change associated with global warming (MEND, 2001 and 2004b). |
[[#top|Top of this page]] | [[#top|Top of this page]] | ||
Revision as of 16:48, 2 June 2009
- 6.1 Introduction
- 6.2 Goals and Objectives of Prevention and Mitigation
- 6.3 Approach to Acid Rock Drainage Prevention and Mitigation
- 6.4 Drivers of Acid Rock Drainage
- 6.5 Phased Approach
- 6.6 Overview of Best Practice Methods
- 6.7 Secondary Impacts
- 6.8 Selection and Evaluation of Alternatives
- 6.9 Design and Construction Considerations
- 6.10 Maintenance and Monitoring Considerations
- 6.11 References
- List of Tables
- List of Figures
6.0 PREVENTION AND MITIGATION
6.1 Introduction
The most recent and widely accepted methods for the prevention and mitigation of ARD are presented in this Chapter 6. Discussions in this chapter include the principles and objectives for prevention and mitigation and definitions and terms, suitability and applications, expectations and limitations, and primary references. While this chapter focuses on environmental technologies, regulatory, social, economic, and sustainability issues must always be managed within the applications of all prevention and mitigation techniques.
As discussed in Chapter 2, sulphide mineral oxidation occurs naturally as part of the sulphur cycle. ARD is formed when sulphide mineral oxidation and acid generation occur at a rate that overwhelms the neutralization processes. In the context of ARD management during mining, the goal of mitigation measures is often to maintain or control the rate of sulphide mineral oxidation so that ARD formation is prevented or reduced to minimal or acceptable levels. Absolute prevention of ARD may require that all reactive sulphide bearing minerals remain virtually isolated from atmospheric oxygen. However, absolute prevention of ARD may not be required for protection of environmental quality.
The basic approaches to prevent ARD are similar at coal mines and hard rock mines: reducing oxygen ingress and preventing contact with water that can act as a transport medium for oxidation products. Coal mines may make use of low-permeability covers, selectively handle the pyritic material and place it high in the backfill so that it will not be exposed to ground water, and/or place it beneath or mixed with alkaline strata or some added alkaline material or by-product (e.g., alkaline fly ash, steel slag), which neutralizes acidity and thereby inhibits the high rates of pyrite oxidation that can occur when the pH gets low enough to permit iron-oxidizing bacteria and ferric oxidation of pyrite to become significant (Brady et al., 1990; Perry and Brady, 1995; Rich and Hutchison, 1990; Rose et al., 1995; Skousen and Larew, 1994; Smith and Brady 1998; Wiram and Naumann, 1995). These latter techniques have also been applied with some modifications at hard rock mines, as will be discussed later in this chapter.
The implementation of methods for prevention and mitigation depends on mine development stage, deposit type, geochemistry, climatic regime, terrain, surface water, geology and groundwater, and aquatic and terrestrial ecosystems. Material availability, land management and land use requirements, receptors, risk, cost, maintenance, and sustainability and regulatory requirements will also influence the approach selected.
Best practice prevention and mitigation methods continue to evolve with new knowledge, experience, and observed performance. Long-term data are now available for many of the approaches to confirm that the methods are either working or not working. Most of the same principles and methods for ARD prevention can be applied to NMD and SD because sulphide mineral oxidation is the primary cause of NMD and SD.
6.2 Goals and Objectives of Prevention and Mitigation
Prevention is a proactive strategy that obviates the need for the reactive approach to mitigation. Mitigation will be the usual initial course of action for an already existing case of mine drainage that is adversely impacting the environment. Despite this initial action, subsequent preventative measures may also need to be considered in the context of reducing future contaminant load, and thus reducing the ongoing need for mitigation controls. For example, the amount of seepage requiring treatment may be reduced if the current source strength is reduced.
For both prevention and mitigation, the strategic objectives must be identified because, to a large extent, these strategic objectives will define the control methods that need to be used. The process of identifying the strategic objectives should consider the following:
- Quantifiable risks to ecological systems, human health, and other receptors;
- Site-specific discharge water quality criteria;
- Capital, operating, and maintenance costs of mitigation or preventative measures;
- Logistics of long-term operations and maintenance; and
- Required system longevity and modes of failure.
ARD/ML prevention is the key to avoid costly mitigation. ARD, NMD, and SD are all the result of natural weathering processes that occur under atmospheric conditions. The primary goal of the prevention is to stop contaminated drainage from leaving the mine site at its source by minimizing reaction rates, leaching, and the subsequent migration of weathering products from mine waste.
Typical objectives for ARD control are to satisfy environmental criteria using the most cost-effective technique. Technology selection should consider predictions for discharge water chemistry, advantages and disadvantages of treatment options, risk to receptors, and the regulatory context, including permitted discharge water quality (see Chapter 9).
6.3 Approach to Acid Rock Drainage Prevention and Mitigation
Prevention of ARD can be achieved through a risk-based planning and design approach that is applied throughout the mine life cycle, but prevention is primarily accomplished in the assessment and design phases. The prevention process aims to quantify the long-term impacts of alternatives and to use this knowledge to select the option that has the least impact. Mitigation measures implemented as part of an effective control strategy should require minimal active intervention and management.
The primary approach to the prevention and mitigation of ARD is to apply methods that minimize the supply of the primary reactants for sulphide oxidation, and/or maximise the amount and availability of acid neutralizing reactants. These methods involve the following:
- Minimizing oxygen supply because of diffusion or advection
- Minimizing water infiltration and leaching (water acts as both a reactant and a transport mechanism)
- Minimizing, removing, or isolating sulphide minerals
- Controlling pore water solution pH
- Maximizing availability of acid neutralizing minerals and pore water alkalinity
- Controlling bacteria and biogeochemical processes
Factors influencing selection of the above methods include the following:
- Geochemistry (i.e., sulphide/carbonate content and reactivity) of source materials and the potential of source materials to produce ARD
- Type and physical characteristics of the source, including water flow and oxygen transport
- Mine development stage – (More options are available at early stages.)
- Phase of oxidation – (More options are available at early stages when pH may be near neutral and oxidation products have not significantly accumulated.)
- Time period for which the control measure is required to be effective
- Site conditions – location, topography, and available mining voids, climate, geology, hydrology and hydrogeology, availability of materials, and vegetation
- Criteria for discharge
- Risk acceptance by company and stakeholders
In general, more options and more effective options are available earlier in the mine life, as indicated in Figure 6-1. More than one measure, or a combination of measures, may be required to achieve the desired objective.
6.4 Drivers of Acid Rock Drainage
The primary drivers of ARD can be classified in three distinct categories of physical factors, geochemical weathering processes, and climate and physical environment. Each is described below in Sections 6.4.1 through 6.4.3.
6.4.1 Physical Factors
The structural nature and physical environment of the ARD source material influences selection of the most appropriate method (or methods) for prevention and mitigation. Typical mining and non-mining related sources are reviewed in Chapter 4. Specific mining related examples include the following:
- Waste rock – coarse, highly permeable unsaturated porous overburden material (boulder to sand size) deposited in the mine pit or as rock dumps above the natural topography
- Tailings and coal refuse – fine, variably saturated or unsaturated porous material (clay to sand size) derived from ore processing or beneficiation, generally deposited in engineered impoundments
- Spent ore and heap leach residues
- Open or filled pits, containing massive and fractured rock
- Underground mine structures, shafts, drifts, and stopes
- Block cave rubble zones (can be transitional between waste rock and fractured rock medium characteristics)
The structural nature and physical environment of each source must be described with respect to the water table, seepage and flow, degree of saturation, oxygen, heat, and solutes to provide a detailed level of understanding of how geometry, hydraulic properties, and structure influence control mechanisms, behaviour, and performance. For example, Figure 6-2 illustrates processes that occur within a waste rock dump and are influenced by structure. By necessity, solutions to prevent ARD will therefore be site specific.
6.4.2 Geochemical Weathering Processes
Factors that control ARD generation are described in Chapter 2. The integration and coupling of ARD factors must be used to assess the best approach to prevention and mitigation of ARD. Controls may be targeted at each aspect of the ARD generation process.
6.4.3 Climate and Physical Environment
- Tropical humid
- Dry
- Mild mid - latitude
- Severe mid - latitude
- Polar
- Highland
A physical environment is formed when water and energy budgets are coupled with the terrain, landforms, surface topography, soils, stratigraphy and geology, surface hydrology, hydrogeology, and flora. Together these factors comprise an “earth system” and need to be considered in developing the most suitable methods for prevention, control, and mitigation of mine discharges.
For example, in open pit hard rock mines, most of the mine wastes are typically stored on the surface and exposed to atmospheric conditions. Because the main source of mine drainage is meteoric water, local climate has a direct influence on the selection of prevention and mitigation methods for ARD. The Köppen system (Peel et al., 2007) is a well known method for climate classification. Methods for prevention and mitigation that are suitable in a tropical humid climate, such as Borneo, Indonesia, may fail in a dry climate such as as the Pilbara region of Western Australia or the polar climate in the Northwest Territories of the Canadian Arctic.
6.5 Phased Approach
- Question
- Hypothesis
- Experiment
- Analysis
- Conclusions
A phased approach to the implementation of methods for prevention, control, and mitigation is recommended. Experience based on direct observation is of great value for decision analysis (adaptive management) and the design of systems for prevention and mitigation. For example, longer-term data for some ARD methods (e.g., water covers) are now available. Time frames associated with mine life cycles are decades and sometimes centuries, so the time frames required for the verification of prevention and mitigation methods may be long.
The observational approach based on scientific methods is the most appropriate approach. The ARD prevention plan should be as robust (based on scientific and engineering methods) and flexible as possible so that it can be adapted based on observed performance. Figure 6-3 illustrates adaptive management and implementation using a phased approach, which begins with the development of hypotheses and conceptual designs based on site characterization and problem definition. The phased process can begin and enter at any stage of mine development. The key step is to develop a system design that leads to the basis for analysis and the capacity to make decisions. The process should include a staged approach that allows ongoing analysis, verification, and improvement in system design. Regional and local experience at nearby mines, where available, should also be used to minimize redundant investigations and to optimize the most successful methods for prevention and control. The phased approach leads to development of a monitoring and maintenance program that reinforces and improves system design.
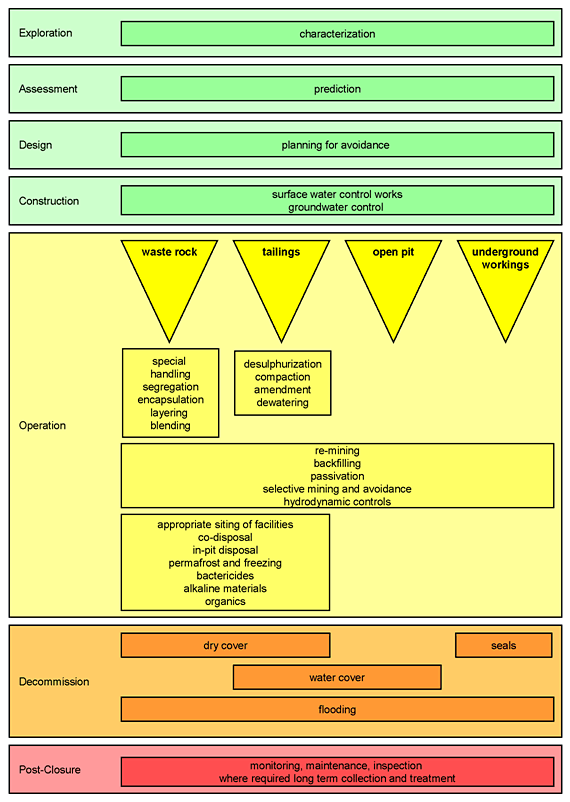
6.6 Overview of Best Practice Methods
This Section 6.6 presents a summary of the methods available for prevention and mitigation of mine drainage, as shown in Figure 6-4. The purpose of this section is to provide an overview of best practice methods. Detailed design manuals are provided in the reference section such as MEND (2001), MEND (2004a) and DWAF (2007),are provided in the reference section.
6.6.1 Avoidance
Sites that generate ARD with a high solute load and concentrations of contaminants can incur significant long-term ARD treatment costs that can impair the economic success and, in some cases, the viability of a project. Measures for ARD prevention, mitigation, and treatment must therefore be included in evaluation of mine lifecycle costs, both for processing wastes that are derived from the ore and overburden or waste rock that must be stripped to access the ore. The result of this overall assessment may be a decision not to mine a particular rock mass at some mines, or to mine in a manner that might initially be thought to be more costly (ADTI, 1998).
Avoidance is not synonymous with non-development in mining. Avoidance includes the decision not to extract a particularly reactive rock type that will be too difficult to manage in the future. This may require the development of mine designs that avoid or work around difficult rock types through alteration of mine access, inclines, stopes, and open pit designs.
6.6.2 Re-mining
Historical legacy sites that are currently generating ARD are some of the most difficult to remediate because significant volumes of reactive wastes may need to be managed or even moved. However, sometimes abandoned surface or underground mines can be re-mined to remove valuable material that remains there. Re-mining provides opportunities to improve waste disposal systems and reduce or remove sulphide minerals.
Re-mining methods used include the following:
- Excavation and milling of waste rock and tailings deposits;
- Covering and burial of existing waste piles with new benign waste;
- Push-back of existing pits walls to excavate and process reactive rock, leaving lower grade non-reactive wall rock
- Excavating areas previously mined by room-; and-pillar methods, extracting the reactive rock along with the remaining commodity (e.g., coal), also called “daylighting”
- Re-handling wastes and moving to improved storage facilities
- Re-handling wastes and moving them to improved storage facilities.
Typically, at coal mines, re-mining and reclamation reduce acid loads by: 1) decreasing infiltration rates, 2) covering acid-producing materials, and 3) removing the remaining coal which at many sites is the source of most of the pyrite. However, re-mining can also expose pyritic overburden strata and so can actually degrade water quality unless supplemental abatement measures, such as alkaline addition, are used (Hawkins, 1998). Therefore, an assessment of re-mining options should consider the potential long-term costs for mitigation and treatment of drainage, vs. the potential for increased recovery of resources. At many site the value gained from extraction can finance further opportunities to mitigate ARD (PaDEP, 1998 Chapter 17).
6.6.3 Special Handling Methods
Specialized handling procedures for mine waste products, including tailings and waste rock, are often adopted as part of a strategy to minimize ARD. This is often the first step in implementing the ARD management plan. The handling procedures are based on the result of the ARD prediction program (Chapter 5). Special handling approaches are discussed in this chapter and in Chapter 9.
6.6.3.1 Incorporation in Mine Plan
Mine waste handling may be incorporated into mine planning to minimize exposure of materials to atmospheric conditions and minimize the volume of material left on surface at closure. Examples of common practices include the following:
- Use of tailings backfill for underground support. This method can also reduce overall costs compared to conventional hydraulic backfill.
- Subaqueous disposal of reactive wastes in mine voids, including placing mining wastes into open pits and underground workings. The economic feasibility of this practice is highly site specific, but is fairly common, and the approaches are well developed. Mined-out pits can provide a void for storage of tailings, waste rock, or seepage water. Pits provide the potential for long-term geologically stable containment while traditional impoundments often require monitoring and maintenance to ensure stability of the constructed dam walls over the long term.
- In-pit disposal of tailings or waste rock may be combined with other strategies, such as subaqueous or underwater disposal, alkaline addition, cover technologies, and sulphate reduction, which are described later in this chapter.
- Minimization of the waste footprint to reduce capping and revegetation costs, or to reduce the surface area exposed to precipitation and oxidation.
- Avoidance of placing waste storage facilities near sensitive receiving environments or regionally significant aquifers.
6.6.3.2 Segregation
Segregation of waste rock (also referred to as selective handling) involves physical separation of PAG and NAG materials (MEND, 2001). While segregation on its own does not prevent ARD, it is often a necessary step within the mitigation plan. PAG materials may be used or placed in engineered configurations to minimize impacts to the receiving environment. Commonly, one attempts to either ensure that PAG material is kept completely saturated (to minimize exposure to air) or to minimize surface and groundwater contact with PAG materials while maximizing groundwater contact with alkalinity generating materials. Segregation of ore and waste is standard mining practice, and similar techniques may be used to separate waste types (see Chapter 9). The development of an ARD management plan that involves segregation generally proceeds as follows:
- The mine waste plan is developed based on detailed geochemical characterization using procedures defined in Chapters 4 and 5 and appropriate models (i.e., block models for open pits).
- Waste management and operational monitoring programs are established to identify and segregate materials before handling, transport, and deposition.
- Special handling procedures are developed, such as cellular construction of waste piles or purpose-built repositories for reactive materials with system design to provide isolation and sealing (e.g., subaqueous disposal of reactive wastes or storage of PAG rock within tailings impoundments). Waste rock dump lifts might be compacted or covered with thin layers of lower permeability material to inhibit infiltration and oxygen transfer.
At coal mines, the PAG material is typically placed on a pad of non-reactive rock so that it is elevated above any fluctuating water level in the pit. The material should be compacted and treated with alkaline material to neutralize the acid-producing potential, capped with a layer of low permeability material, and then covered with non-acid-producing material and topsoil to reduce water and air movement into the acid rock. Further information on special handling at coal mines may be found in Perry et al. (1998: http://www.dep.state.pa.us/dep/deputate/minres/districts/cmdp/chap14.html), Skousen and Ziemkiewicz (1996), and Hawkins et al. (2004).
6.6.3.3 Tailings Desulphurization
Sulphide separation, or depyritization, of tailings can reduce or eliminate ARD. Flotation circuits are used for sulphide separation to remove reactive sulphides to produce relatively low volumes of pyrite concentrates, which can then be contained in specifically designed disposal cells or placed into flooded mine workings (i.e., underground and open pits).
Depyritized tailings with non-acid generating or acid consuming characteristics may be stored in large volume repositories. Relatively clean tailings materials may also be used for other prevention and mitigation methods, such as soil covers (Sjoberg-Dobchuk et al., 2003). However, the acid generating characteristics of the depyritized tailings should be verified to ensure that it is not acid generating before it is used as a cover material.
In general, the feasibility of this sulphide separation option depends on the characteristics of tailings and therefore must be assessed on a site-by-site basis. The flotation processes must be sufficiently effective to remove enough sulphide minerals to render the remaining tailing nonacid generating. The cost of the process may be offset by the production of a relatively clean tailings material that can potentially be used to provide a final cover layer, rather than having to mine or import material from elsewhere (MEND, 2001; Bussière, 2007).
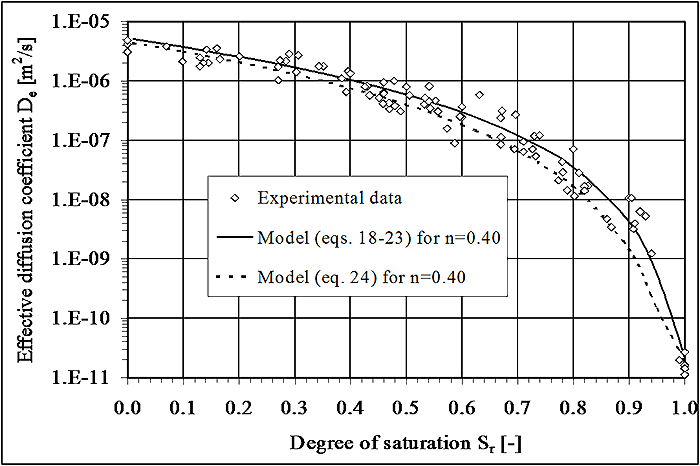
6.6.3.4 Compaction and Physical Tailings Conditioning
Control of physical properties of tailings may be accomplished by methods including thickening, filtration, compaction, and gradation control. The purpose of conditioning is to improve physical properties, such as reduction of hydraulic conductivity, to limit the ARD process. For example, a decrease in porosity may result in a decrease in both hydraulic conductivity and oxygen diffusion. Figure 6-5 (Aubertin, 2005) illustrates the relationship between the coefficient of oxygen diffusion and degree of saturation for soils or porous media. It illustrates that oxygen diffusion rapidly decreases by 3 to 4 orders of magnitude as the degree of saturation increases above 85%. The concept described here has been successfully implemented at a number of sites, including the multi-layered cover design at the LTA site in Quebec, Canada described by Bussière (2007).
Traditional disposal of tailings slurry to impoundments by hydraulic deposition typically produces a beach of coarser material near the spigot and finer materials further from the spigot. The beach materials are usually above the water table and are unsaturated and therefore prone to oxidation. Sulphide segregation may occur on beaches due to the higher specific gravity of pyrite vs other non-sulphide minerals including carbonates. Finer tailings tend to have lower permeability, water retaining capacity and resistance to air entry, and are more likely to be saturated. However, finer materials are often unconsolidated and prone to liquefaction and settlement that make reclamation more difficult.
For geotechnical reasons, the embankments for tailings impoundments are generally designed to be well drained and unsaturated. Construction of embankments with sulphide-bearing tailings may pose significant ARD risks compared to construction with inert materials.
Several methods are available to improve physical properties of tailings (Bussière, 2007). Tailings can be thickened (removal of water) to produce a non-segregating material or paste (ACG, 2006). Selection of thickening technology depends on tailings geochemical and physical characteristics and must be reviewed on a site by site basis. Compaction of placed materials will decrease oxygen diffusion and void ratios, decrease water permeability, increase water retention, and increase saturation levels. Installation of wick drains or blast densification can achieve the same result, depending on the tailings deposit.
Layering tailings deposits with clean or low sulphur layers may decrease oxygen gradients and limit oxygen diffusion. Tailings gradation and fines content may be adjusted to create fine layers that maintain high saturation (Figure 6-5) and restrict oxygen flow to underlying sulphide-bearing coarser layers. This method of layering is best implemented as a closure approach to create a cover, and may be achieved through controlled tailings discharge management and deposition planning.
6.6.3.5 Encapsulation and Layering
Encapsulation and layering involves placing acid producing and acid consuming materials (typically waste rock) in geometries designed to control or limit ARD. The effectiveness of the encapsulation and layering is governed by availability of materials, the general balance between acid producing and acid neutralizing materials, the type and reactivity of acid-consuming material, deposit geometry, the nature and flow of water through the deposit, and chemical armoring of alkaline materials (MEND, 1998a and 2001; Miller et al., 2003 and 2006). Layering of waste rock and tailings materials is discussed below in Section 6.6.3.7.
6.6.3.6 Blending
Blending is the mixing of waste rock types of varying acid generation and neutralization potential to create a deposit that generates a discharge of acceptable quality. The effectiveness of blending as an option depends on the availability of materials and the mine plan, the general stoichiometric balance between acid producing and acid neutralizing materials, geochemical properties, reactivity of waste rock types, flow pathways created within the deposit, and the extent of mixing and method of blending. Homogeneous and thorough mixing is generally required to achieve maximum benefit (MEND, 1998a and 2001). Evidence from field trials indicates limited success with mixing of waste rock types using haul trucks, but better mixing of waste rock with limestone was achieved using a conveyor system and stacker (Miller et al., 2006).
Operational experience indicates that, for effective blending of PAG rock with limestone, it is essential that all size fractions within the blend be at least acid-base neutral (i.e., NPR (ANC/MPA) of at least 1). Since the run of mine particle size of limestone is generally coarser than PAG rock, the acid base balance of the bulk waste rock needs to be greater than balanced. Experience with rock types at Freeport (Indonesia) and Ok Tedi (Papua New Guinea) indicate that well mixed PAG and limestone rock needs to have a bulk NNP of more than 150 kg CaCO3/t (or NAPP less than 150 kg H2SO4/t). The actual blend will depend on site factors and particle size distribution for each rock type.
6.6.3.7 Co-disposal
Co-disposal is the disposal of waste rock with tailings. Co-disposal can take several forms, which vary depending on the degree of mixing, as summarized in Table 6-1 (Wickland et al, 2006).
Co-disposal has the potential to limit ARD as follows:
- Co-disposal of highly reactive waste rock within saturated tailings impoundments
- Layered co-mingling of thickened tailings and waste rock
- Thorough mixing of paste or filtered tailings with waste rock to create “paste rock”
For ideal mixing, the void space of waste rock particles are filled with finer tailings particles which typically have a higher moisture content, thereby limiting the flow of oxygen and water relative to deposits of waste rock alone. Amendment of tailings with alkaline materials is possible, and co-disposed materials will have a lower rate of ARD production than only blending of waste rock types. Benefits and considerations for co-disposal are listed in Table 6-2.
| Additional Benefits | Considerations |
|
|
Pumped co-disposal of coarse and fine coal beneficiation wastes has been implemented at coal mines in the United States of America, Australia, and Indonesia since the 1990s (Williams et al., 1995), though pumped co-disposal typically results in a segregated deposit. Co-disposal is used in South African coal operations where coal slurry is placed within a dam constructed of coarse reject. However, mixing waste rock and tailings for blended co-disposal and paste rock is in the research stage, and has not been implemented at full scale at operating hard rock mines.
6.6.3.8 Permafrost and Freezing
Permafrost or ice covers approximately 25% of the earth’s surface, and the mine operator may take advantage of cold conditions to control weathering and drainage. Permafrost is generally defined as ground that remains below 0º Celsius for more than 2 years. The term permafrost does not imply ice or water content.
Freezing of materials to control acid generation has been used as a strategy to control ARD at several cold sites (MEND, 2004b). Chemical activity does not stop at 0º Celsius, and freezing point depression because of chemical content of pore water may result in unfrozen water present in mine waste materials at temperatures well below freezing.
Periodic warming of the surface during summer periods in zones of permafrost results in thaw within an upper “active zone.” Strategies for limiting chemical weathering may include a cover that prevents penetration of the active zone into PAG materials so that the PAG materials remain frozen (MEND, 2009).
Tailings deposition planning may be optimized to promote freezing during winter months and limit thawing during summer months. Thermal analysis is required to predict long-term freezing of reactive materials. Experience has shown that tailings will freeze on cold sites, such as at the Nanisivik Mine, Nunavut, Canada (Claypool et al., 2007).
As a general rule, permafrost encapsulation requires approximately -8º Celsius mean annual air temperature. One possible limitation of relying on freezing alone is the potential for climate change associated with global warming (MEND, 2001 and 2004b).
6.6.4 Additions and Amendment Methods
Methods for use of amendments and additions are described below in Sections 6.6.4.1 through 6.6.4.4.
6.6.4.1 Passivation
Passivation is the treatment of reactive rock surfaces to limit release of contaminants by installing a chemically inert and protective surface layer. For example, for permanganate passivation of sulphidic rock, potassium permanganate is applied at a high pH (> 12) in the presence of magnesium. Oxidative reactions on pyrite create a manganese-iron-magnesium layer on the surface that prevents further oxidation of the pyrite.
Passivation using potassium permanganate is in the research stage and has not been applied at a full-scale mine. Pilot-scale experiments have shown that passivation can substantially reduce contaminant release for more than 5 years but the long-term stability of this treatment still needs to be established. Treatment of freshly mined surfaces has shown the greatest success and requires the lowest consumption of reagents compared to treatment of aged reactive rock surfaces, which often have high acidity and contain an oxidation rind that limits the effectiveness of passivation (Miller and Van Zyl, 2008).
6.6.4.2 Alkaline Materials
A relatively common approach to mitigation of ARD is the control of solution pH by the addition of alkaline materials. Methods for use of alkaline materials include blending with waste rock, amendment of tailings, placement of alkaline material above or below wastes as liner or cover materials, and treatment of drainage (BC AMD, 1989; PaDEP, 1998, Chapter 13; Miller et al., 2003 and 2006; and Taylor et al., 2006).
Addition of alkaline materials can control ARD provided intimate blending can be achieved; this is often a difficult task. The effectiveness of the method is dependent on the pathways of movement of water through the system, degree of mixing, and the nature of the contact between acidic rock and the alkaline materials. If the mixture is not homogeneous and there is not good contact between the materials, then localized “hot spots” of acid generation may occur. The type, purity, reactivity, availability, and proportion of the alkaline material are also important. Common additives include limestone (CaCO3) and lime (CaO or Ca(OH)2). Liquid forms are considerably diluted relative to solid forms and have limited longevity, but may provide better penetration of the acid generating mine waste.
Use of alkaline materials overlying acid generating mine waste can provide a long-term source of alkalinity, may lead to formation of a “chemical cap” (i.e., a hardpan) at the interface between alkaline and acid generating material, and may produce a passivating coating on the surface of acid generating particles. However, the effectiveness of this approach depends on the solubility of the neutralizing agent and the flux of infiltrating water available to carry the alkalinity down to the underlying sulphidic waste. Use of alkaline materials underneath acid generating mine waste may result in formation of a passivating coating on the surface of alkaline particles, thus reducing considerably the effectiveness of this strategy. Benefits and limitations of alkaline amendments are summarized in Table 6-3.
| Configuration | Benefits | Limitations |
|
|
|
|
|
|
|
|
|
|
|
|
6.6.4.3 Use of Organic Matter
Organic materials can be mixed directly with wastes to consume oxygen and promote metal reduction in an anoxic environment by naturally occurring bacteria. The bacteria reduce available sulphate and create insoluble metal sulphide precipitates. Examples of organic substrates include sewage sludge, municipal landfill waste, and pulp and paper waste. Similar concepts are described as a passive treatment method in Chapter 7. The method is limited by exhaustion of organic materials. Use of organics as part of a cover design is discussed below in Section 6.6.7.3.
6.6.4.4 Bactericides
Bactericides are amendments applied to acid-producing materials to control the growth of bacteria that catalyze the ARD process. Bactericides do not stop or eliminate ARD, but can slow the rate of reaction. They are not effective for highly reactive wastes. Chemical weathering processes continue without bacteria present. Bactericides work best on fresh or unoxidized sulphides, can be applied in a liquid or spray form, and are commercially available. Examples of bactericides include anionic surfactants such as sodium laurylsulphate, benzoate compounds, sorbate compounds, and phosphate compounds. Sodium laurylsulphate has been used to treat coal mine wastes.
Bactericides may be used in combination with other methods (USEPA, 2003 and BC AMD, 1989). Bactericides have had very limited application at hard rock mines because of the generally highly permeable nature of waste rock.
6.6.5 Water Management Methods
Methods for water management include diversion of site surface drainage and groundwater. Water acts as a transport mechanism and a reactant. However, the amount of water required for sulphide oxidation is virtually always present (except in extremely arid environments) in excess. The primary role of water management is to reduce infiltration and thereby reduce the volume of contaminated drainage. It is generally much more cost effective to treat a smaller volume of more contaminated water than a much larger volume of less contaminated water that still fails to meet discharge standards. Water management should be assessed at the local watershed scale (DWAF, 2006).
6.6.5.1 Hydrogeological and Hydrodynamic Controls
Hydrogeological controls for groundwater systems include both barriers and higher permeability features. The objective of hydrogeological controls is to control the flow of groundwater. The use of hydrodynamic control options is site specific and depends on site conditions, including climate, topography, geology, hydrology, and hydrogeology.
An example of hydrogeological containment is disposal of tailings in an open pit with a pervious surround. A granular filter layer is placed between the pit wall and the low-permeability tailings to provide a high hydraulic conductivity pathway for regional groundwater flow. Because the tailings have a lower hydraulic conductivity than the surrounding granular layer, groundwater preferentially flows around, rather than through, the tailings (e.g., Rabbit Lake Mine, Rabbit Lake, Saskatchewan, Canada).
6.6.5.2 Dewatering
Dewatering involves lowering the groundwater table to change the hydraulic gradient. Cleanup gradient groundwater is collected before encountering reactive wastes. Examples include pit dewatering to reduce seepage through pit walls and upgradient shallow groundwater collection ditches above tailing ponds and waste rock dumps. The collection of contaminated water using groundwater pumping is considered part of treatment (see Chapter 7). Conventional hydrogeology science is applied to design control measures, such as well spacing and pumping rates.
6.6.5.3 Diversion
Control of surface water can minimize flow through PAG materials and reduce ARD production for both surface and groundwater systems. Surface water diversion may include upstream ditching or impervious channels to divert drainage across impacted areas. Drainage works must be sized based on catchment hydrology, including snowmelt and storm events, and will typically require ongoing maintenance (because of debris accumulating, sloughing, and animal activity) to ensure long-term performance. Therefore, the best long-term solution often involves selection of a disposal site, which minimizes the need for surface diversion works (ADTI, 1998).
Control of groundwater may include hydraulic mine seals in underground workings. Exploration boreholes can be a significant source of groundwater flow that can be controlled by proper grouting following drilling. Conventional grouting methods apply to fractured rock but complete sealing may be difficult because water flows might still migrate using alternative lower permeability pathways. Grouting may also result in the buildup of large pressure-heads that have safety implications in underground mines, especially where the host rock contains karst features. French drains and soil-bentonite-slurry cut-off walls may be used in surface soils.
Control of groundwater in groundwater discharge areas may be difficult to achieve and maintain. This might be best addressed by choosing a site for waste disposal that is not a groundwater discharge zone if groundwater is a significant issue with respect to performance of the prevention/mitigation approach (DWAF, 2006; ADTI, 1998; and TEAM NT, 2004). Soil covers may divert water and limit infiltration, and are discussed in Section 6.6.7.
6.6.5.4 Flooding
Flooding of underground or surface mine voids with water has the potential to significantly inhibit the supply of oxygen so that ARD production is not a concern – see Section 6.8.7.1. The scientific basis for the approach is also provided in MEND (2001). However, caution must be used with flooding of previously oxidized wastes because stored oxidation products may be dissolved during the initial inundation of pits, wastes, or workings.
Factors to be considered for flooding include the status of the mine plan and schedule of waste production, potential for mobilization of stored oxidation products, availability of open workings or pits, and capacity to store waste products (note that mined material will increase in volume by approximately 25% to 30% [swell factor]). ARD from block caves and glory holes may be difficult to manage as access may be restricted. However, flooding may be possible depending on bottom drainage conditions.
6.6.5.5 Seals
When decommissioning an underground mine, knowledge of the areas within the mine that are geochemically most reactive and knowledge of water ingress and discharge locations will enable design and implementation of a rational ARD management plan aimed at controlling the flow of water to minimize water quality deterioration. This process would involve construction of seals and also perhaps reinforcing of some areas in advance of flooding to accommodate water flow. Use of seals and reinforcements is a good example of prevention and minimization by design.
Hydraulic seals limit movement of air and water through mine workings. Seals can be used to promote flooded conditions. Flooding of mine workings may generate considerable hydraulic head and seals and, therefore, require rigorous engineering design. An example case study for underground mine flooding is described in Lang (2007) for the Millennium Plug installed at the Britannia Mine in British Columbia, Canada. For the Britannia Mine, a concrete plug was placed within a tunnel to prevent effluent from being released to the environment and caused flooding of the mine to prevent oxygen entry and further acid generation.
Sealing of drifts, adits (horizontal), and stopes (angled mine workings) is common when decommissioning an underground mine. Seal construction may include brick and mortar walls to contain concrete pumped between the seals. Horizontal seals or plugs can be left with through piping that may be opened to allow drainage of mine waters, if necessary. Where safety of workings is a concern for the construction of seals, particularly in old mine sites, concrete seals can be placed remotely through drill holes. Seals at active mines are rarely constructed remotely.
Three common and successful approaches to constructing seals include pressure grouting of adjacent ground, increasing the length of the seal to increase the seepage flow path length, and installation of secondary seals (ADTI, 1998 and ERMITE, 2003).
6.6.6 Liners
Low permeability liners may be required to maintain saturated conditions in the overlying waste or to protect underlying groundwater resources. Various approaches are described in more detail below in Section 6.6.7.
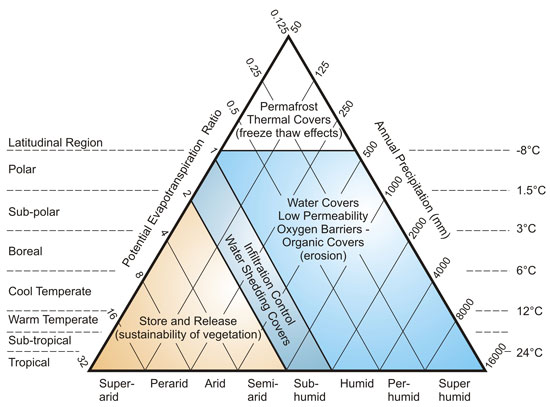
6.6.7 Dry Cover Methods
Dry covers are typically earthen, organic, or synthetic materials placed over mine wastes. In the long term, these cover systems must interact with climate, hydrology, human activity, vegetation, animals, biogeochemistry, and settlement of underlying material. Figure 6-7 provides general guidance on appropriate cover types for climate type. Figure 6-7 shows a tri-linear plot of climate classification, rainfall, and biotemperature. The regions inside the plot are divided into zones for the suitable application of various cover types, depending on climatic conditions ranging from arid to high rainfall and tropical to polar.
6.6.7.1 Soil Covers
Soil covers consist of granular materials placed over mine wastes to reduce or control ARD production (MEND, 2001). Appropriate cover systems are dependent on climate type, as illustrated in Figure 6-7. Oxygen barrier covers with low water permeability prevent oxygen entry by maintaining high water saturation. These covers are best suited to climate regimes where the potential evapotranspiration to precipitation ratio is less than 1.0. It is difficult to maintain saturation in dry climates where the potential evapotranspiration ratio is greater than 1.0; therefore, the design objective for the cover becomes the minimization of infiltration through water diversion, runoff and shedding, or store and release mechanisms.
Single soil layers were originally used in Canada for revegetation of mining wastes and it was hoped that they would reduce ARD. In general, they were not effective. Engineered multilayer soil covers for ARD control became popular in the 1990s with the development of the science of unsaturated media that enabled prediction of evaporation from soil cover systems (e.g., the SoilCover Model). Multilayer systems in wet climates often include a relatively loose layer for vegetation roots, a compacted fine grained layer that maintains a high moisture content to reduce oxygen transfer, and a coarser capillary break that prevents upward migration of soluble salts from the underlying mine wastes. In general, compacted clay rich barrier cover designs function best in wet tropical and temperate climates. Store and release covers perform best in wet/dry climates with high potential evaporation equal to 2 or 3 times precipitation. It is possible to also develop hybrid store and release cover over a compacted layer or even a geosynthetic clay liner (GCL). Recent reviews based on 10 to 15 years of cover performance data indicate that covers may limit, but do not stop, infiltration and ARD generation (Wilson 2008a; Wilson et al., 2003; and Taylor et al., 2003). However, the achieved reduction in ARD and metal release may be sufficient to meet design goals and at a minimum would reduce water treatment requirements.
Key objectives for multilayer soil covers include the following:
- Limitation of oxygen ingress – typically by including a saturated zone (i.e., fine grained compacted soil layers designed to achieve high levels of saturation greater than 85%) to minimize oxygen diffusion as shown in Figure 6-5
- Limitation of infiltration – either by a low-permeability layer or a moisture storage and release layer
- Preservation of runoff water quality
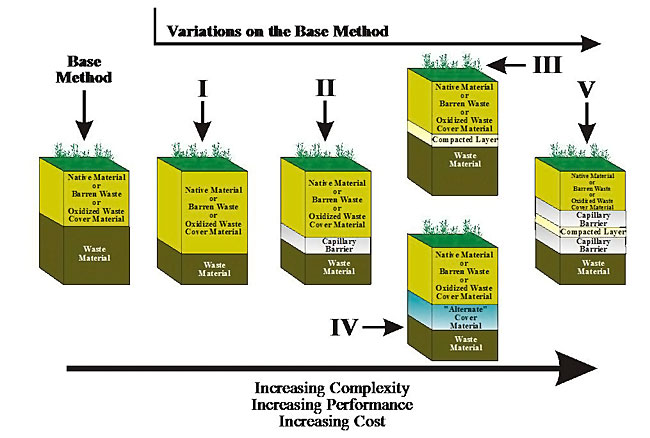
Soil cover designs are site and climate specific and generally require significant quantities of suitable material for construction, which may be a limiting factor in their implementation (i.e., high transport cost of the materials). Cover configuration will vary with the design objective. Soil layers can be designed to include a capillary barrier where a fine soil overlies a coarser soil. The finer soil is designed to be tension saturated for impeding oxygen flow. Capillary barriers may also be used to reduce infiltration. Design manuals for soil covers include MEND (2004). Recent studies examined the effectiveness of covers on a watershed scale (MEND, 2007).
Examples of soil cover designs are shown in Figure 6-8 (MEND, 2001). Considerations for use of soil covers are listed in Table 6-4 and an excellent similar compilation is provided in MEND (2004).
| Considerations | Limitations |
|
|
6.6.7.2 Alkaline Covers
Alkaline cover materials, such as limestone, placed over PAG materials can increase alkalinity of infiltration, thereby providing pH control (see Section 6.8.3.6 and Section 6.8.4.2). Alkaline infiltration may react with and generate a surface coating on sulphide bearing materials that isolates sulphide minerals (Miller et al., 2003) and form a hardpan (i.e., “chemical barrier”) at the contact between the alkaline material and the reactive mine waste. Use of the method must consider climate, availability of alkaline materials, geometry and reactivity of alkaline materials, and time of consumption. Infiltration through a limestone cover may transport sufficient alkalinity to neutralize the uppermost portion of the underlying waste, but it is unlikely to prevent ARD unless the underlying waste has an extremely low sulphide content. In order to generate even modest alkalinity (in the range of 60 to 80 mg/L), rainwater needs to be in contact with limestone for about 11 hours. The required thickness of limestone to provide adequate retention time can be 3 to 5 meters for high rainfall climates.
6.6.7.3 Organic Covers
Organic materials are placed over PAG wastes to provide the following:
- A saturated layer that serves as a physical barrier to oxygen
- An oxygen consuming layer – (Decomposition of organic material may create a large biological oxygen demand [note: may need replenishment].)
- Chemical inhibition – (Decomposition products and compounds within the organic material may inhibit the growth and metabolism of acidifying bacteria.)
- Chemical amelioration – (Organic compounds may create conditions that support the reductive dissolution of iron oxides and subsequent precipitation in the form of sulphides thus reducing acid production by ferric iron [see Chapter 2])
- A carbon source for sulphate reducing bacteria
- Limitation of water infiltration by lowering hydraulic conductivity
Organic materials have included pulp and paper residues, sewage sludge, bark, sawdust, sanding dust, fiberboard, pulpwood, deinking residues, peat, compost and carbonaceous matter, or waste rock rich in organic matter.
Organic covers has been shown to reduce acidity but without stopping ARD. Limitations to the process include availability of organic materials for cover, longevity (organic materials will become resistant to decomposition with time), and climate (humid climates may be required to maintain anaerobic conditions in the organic medium).
6.6.7.4 Covers of Sulphide-bearing but Net Neutralizing Materials
Mineral wastes that contain sulphides, but which have an excess of neutralizing potential, can be used as a cover that will consume oxygen but not contribute to ARD generation.
6.6.7.5 Synthetic Covers
Use of synthetic materials to cover wastes can dramatically reduce infiltration. Synthetics include different types of plastics (polyethylene (PE), high density polyethylene (HDPE), chlorinated polyethylene (CPE), chlorosulphonated polyethylene (DuPont trade mark HYPALON), polyvinyl chloride (PVC), linear low-density polyethylene (LLDPE), geosynthetic clay liners, and geomembranes impregnated with bitumen. Synthetics are often subject to degradation by sunlight and must be protected with a cover material. A suitable bedding material layer (for example sand) must be laid in advance of application of the synthetic layer to prevent rupturing by underlying rock. Similarly, the synthetic layer must be carefully covered with a protective overlying layer before adding the final growth substrate or rock mulch layer. Sample configurations of synthetics in soil covers are illustrated in Figure 6-9. Benefits and disadvantages of synthetic covers are listed in Table 6-5.
| Benefits | Disadvantages |
|
|
6.6.7.6 Gas Barriers
Flooding of underground mine workings with deoxygenated air (e.g., nitrogen) could prevent ARD, but applications are relatively rare. Mine ventilation is controlled during operation and any post-closure investigations must consider safe work procedures for confined spaces. For example, atmospheric levels of radon may increase (e.g., Lisheen Mine, Ireland). See Chapter 8 for reference to the extreme safety hazards posed by deoxygenated air as illustrated by fatalities of an environmental monitor and two paramedics in a sampling shed at the Sullivan Mine in British Columbia, Canada.
6.6.7.7 Vegetation
Establishment of vegetation is often included as a criterion for closure. The purpose of the vegetative cover may include erosion control, enhancement of evapotranspiration as part of a store and release cover system, reestablishment of sustainable ecosystems, and satisfaction of requirements for post-closure land use, including regulatory requirements and visual appeal. Depending on leaf area index, vegetation may increase actual the evapotranspiration rate up to a maximum equal to potential evaporation. The function of vegetation for store and release covers is important because it controls performance. It is not possible to implement a store and release cover without vegetation because evaporation from bare ground is extremely low. The overall performance of the vegetation cover is dependent on the cover density, the species composition, and the available rooting depth. Generally, a diverse vegetative community that mimics or replicates the existing native communities in the surrounding area will provide the best long-term cover performance. A cover that is too thin will not only limit the volume of water that can be stored during wet periods, but will also limit the types of vegetation that can become established.
Effects of vegetation must be considered in engineered soil cover design with respect to ARD. Vegetation may physically alter cover systems by way of holes because of roots, tree throw, or blow down, and may uptake and transport contaminants from below the soil cover. Root penetration of cover systems may effectively bypass capillary break layers and provide a pathway to the surface ecosystem. Root exudates and decomposition products create soil structure that increases the permeability of clays. This may have undesirable consequences for water penetration and gas exchange. Long-term maintenance requirements must consider effects of vegetation. Vegetation growth will increase organic content of the cover, and its decay will consume oxygen (see Section 6.8.6.3).
Many jurisdictions encourage the use of local or native species to ensure ecological continuity with surrounding areas and minimize care and maintenance. However, seed of native species may not be readily available or they may be costly. Also, native species may be difficult to establish and lack the grazing resistance and erosion control properties of agronomic species.
6.6.7.8 Landform Design
The long-term integrity of dry soil cover systems must consider the effects of climate and extreme climatic events, hydrology, animals, vegetation, and biogeochemistry. The closure landform should evolve or develop into a condition that is in steady-state harmony with its surroundings. The rate of evolution and the desired end point must be included as goals for reclamation. Cover systems that shed water might increase the potential for erosion and therefore the specified physical design parameters (e.g., slope angle and length) must take into account the variability of the local climate (MEND, 2007). Some ongoing maintenance may be required for at least a period of time after closure.
6.6.8 Water Cover Methods
Disposal of acid generating materials below a water cover is one of the most effective methods for limiting ARD generation. In water, the maximum concentration of dissolved oxygen is approximately 30 times less than in the atmosphere. More importantly, the transport of oxygen through water by advection and diffusion is severely limited relative to transport in air. For example, the diffusive transfer of oxygen in water is on the order of 10,000 times slower than diffusive transfer in air. Results of field and laboratory testing have confirmed that submergence of ARD generating materials is one of the best available methods for limiting ARD generation over the long term (MEND, 2001). Other mechanisms associated with water covers include sulphide reduction by bacteria, metal hydroxide precipitation, and development of sediment layers, which inhibit interaction between tailings and overlying waters. Engineered structures which retain water (dams) are potentially higher risk structures that must be monitored, maintained, and inspected over the long term. For example, regulatory requirements in British Columbia, Canada, and elsewhere include regular performance review of tailings structures.
6.6.8.1 Subaqueous Disposal
Water covers limit the exposure of PAG materials to oxygen. Example configurations for subaqueous tailings disposal are shown in Figure 6-10. Generally, sufficient depth of water over the PAG material must be provided to account for mixing of the water column and to prevent resuspension of wastes by wind or wave action. Water covers may not be suitable for material that has already appreciably oxidized. The “cut off” point at which this distinction is made will be mine-waste specific. General processes for water covers are illustrated in Figure 6-11. Sediment layers can help isolate subaqueous wastes and adjustment of water cover chemistry is also possible.
Requirements for a water cover include a climate with a positive water balance (indicated in Figure 6-7), long-term physical stability of containment facilities and outlet structures (with sufficient capacity to handle extreme events), and water depth sufficient to prevent resuspension by wind and wave action. Designs must consider the potential for periods of extended drought and exposure of previously saturated material. The considerations for a water cover are summarized in Table 6-6. While only thin water covers are needed to effectively prevent oxygen diffusion, a thicker cover (typically 3 meters deep) is needed if preventing resuspension of fine tailing due to wave action is a consideration. MEND (1998) provides a thorough guide for the design of subaqueous impoundments. A distinction is made between use of natural water bodies or flooded mining voids and engineered tailings dams and manmade lakes. Table 6-6 presents a very high-level overview of possible factors that could be considered in the evaluation of subaqueous disposal; the overview in Table 6-6 is not meant to be comprehensive. The proposed use of natural water bodies in particular requires extensive studies of baseline conditions and potential impacts.
| Considerations for Use of Natural Water Bodies (lakes or oceans) | Considerations for Engineered Water Retaining Structures (dikes and dams, pits, underground workings) |
|
Proximity to Mine
|
Dams and Dikes
Pits and underground workings
|
6.6.8.2 Partial Water Cover
The partial water cover concept involves an elevated water table that maintains saturation throughout the bulk of the tailings profile (Bussière, 2007 and Ouangrawa et al., 2006). A surface pond does not extend to the wall or diking along the perimeter of the tailings impoundment, but a small pond may be maintained in the centre to maintain an elevated water table over the entire region of the tailings impoundment. The objective of the partial water cover is to minimize the higher risk of structural failure associated with having a water cover and pond adjacent to the dam wall while maintaining saturation through enough of the waste to limit the maximum extent of oxidation that will occur. The partial water cover method is well suited for operations where two types of tailings are being generated: the high sulphur concentrate produced by desulphurization (see Section 6.6.3.3) that is stored at depth and the nonacid generating tailings that are used as cover above the level of the pond (Sjoberg and Wilson, 2003). A partial water cover is also well suited to the case where the underlying nonoxidized tailings have sufficient neutralizing capacity to assimilate the entire acid load that is produced from the overlying rind of unsaturated tailings. A key design consideration is to raise the water table above the acid generating material by placing nonacid generating material as a cover, controlling the water level by the pond spillway elevation, or both placing nonacid generating material and controlling the water level.
Use of the partial water-cover method must consider climate, topography, hydrology and hydrogeology, the residual neutralizing capacity of unoxidized tailings, and the water characteristic retention curve of the tailings material.
6.6.8.3 Wetland Covers
A wetland or bog cover includes soil, vegetation, and water overlying acid generating wastes. Soil ameliorates extreme climatic drying events and vegetation helps prevents erosion. Water limits oxygen ingress and plants offer passive treatment opportunities (ERMITE, 2003). The most critical operational aspect of using the wetlands and bogs concept are that oxygen depleted and reducing conditions are maintained at the base of the cover profile, thus not only protecting underlying unoxidised material but also creating the potential for precipitation of existing ARD products as sulphides. MEND (1993) also provides a comprehensive case study of a wetland cover.
6.6.8.4 Attenuation
Attenuation measures are discussed in Chapter 7.
6.6.8.5 Streamflow Regulation
Control of surface water flow and drainages that discharge to adjacent receiving streams must satisfy compliancy criteria established by regulatory agencies and internal corporate standards. In most cases, criteria are defined in terms of concentrations for specified parameters (e.g., pH, acidity, alkalinity, metals, sulphate, and major ions), streamflows, and environmental loadings. Concentrations may also be defined based on periods for high flow and low flow.
Assessment of mine drainage often involves development of a comprehensive water balance (i.e., both surface and groundwater) combined with mass loadings. Key components of a surface water balance include precipitation (both daily and hourly), evapotranspiration, runoff, infiltration, and drainage rates from surface structures (i.e., waste rock). Surface water management is controlled most strongly by precipitation, which is highly responsive within short time periods and can be easily monitored with instruments and flow gauges. Contamination of groundwater resources and the migration of plumes to surface water streams are frequently overlooked and can only be evaluated based on an understanding of groundwater hydrogeology and chemical analyses (see Chapter 8). Stormwater management associated with extreme climatic events is often the most important issue for peak flow prediction, design of impoundment storage capacity, freeboard, spillways, flow concentrations, and diversion channels. Design inadequacies and failure of surface water management systems generally occur during extreme events so designs are based on storm return periods and hydrologic assessments. In many cases, it is better to use multistaged designs based on operational flow rates supported with bypass spillways and diversion channels to handle extreme high-flow conditions. Criteria for bypass flow must be established based on risk, peak loadings, and downstream dispersion and dilution. Designs need to minimize the risk of severe erosion and structural stability of major containment facilities, especially after closure.
6.6.8.6 Water Recycle and Reuse
Minimization of water use and water losses is a critical objective, especially in arid climates. Key design options include minimizing process water discharged to tailings (i.e., thickening), use of low permeability liners and barriers, recycling of process waters along with any contaminated discharges and seepage to the mill, and the use of surface water retention ponds for evaporation (climate permitting). Treatment sludges are also frequently discharged to tailings impoundments and may be sent to voids for deep disposal in pit lakes for long-term management.
Salt budgets may also be critical at arid sites for pit lakes and surface impoundments where a negative water balance because of low precipitation and high evaporation can cause evapoconcentration or hyper saline conditions to develop with time.
6.7 Secondary Impacts
Secondary impacts as a direct consequence of prevention and mitigation as well as downstream impacts are discussed in Chapters 8 and 9.
6.8 Selection and Evaluation of Alternatives
No universal solution exists for the prevention, control, and mitigation of ARD, NMD, and SD. While submergence is clearly the most geochemically-stable approach, subaqueous disposal of tailings and waste in natural water bodies, such as lakes or marine environments, can be contentious. The applicability of technologies to ARD sources and phase of mining is shown in Figure 6-4.
Specific evaluation of methods for prevention and mitigation of ARD requires a clear definition of objectives and defined purpose based on the strategic process summarized in Figure 6-1 to Figure 6-12. The specific environmental technologies and options that will work best will be site specific, often governed by climatic considerations. The applicability of several methods described in the preceding sections is summarized in Table 6-7. Some methods have been demonstrated to be effective at many sites around the world while others have had limited demonstration.
Water covers are a proven performer from a geochemical perspective but are sustainable only in climates with a positive water balance (i.e., precipitation > evaporation). In climates with a suitable water balance, geotechnical and long-term hazards with respect to stability, extreme storms and floods, spillways, erosion, and other natural hazards such as seismic events must be considered.
and Climate Considerations
Oxygen Limiting |
|
| Widely Demonstrated | Limited Demonstration |
|
|
Water Limiting |
|
| Widely Demonstrated | Limited Demonstration |
|
|
Geochemical |
|
| Widely Demonstrated | Limited Demonstration |
|
|
| (A) Tropical humid | (D) Continental severe mid-latitude |
| (B) Dry | (E) Polar |
| (C) Temperate mild mid-latitude | (H) Highland |
In-pit disposal and mine backfill might be preferred in some situations, but this method of disposal usually only becomes available after or well into the mine operating phase.
In many cases, more than one approach or method will be required. For example, sulphide separation combined with water covers, elevated water table, and barrier covers may prove to be the best combined system for a given tailings impoundment.
Cost and economic viability must also be evaluated and similar to the other criteria for environmental and social settings, costs are usually site specific. Cover systems for tailings and waste rock deposits are usually costly. Although costs vary widely, soil covers costs can be approximately $100,000 per hectare (ha). The application of synthetic and complex multilayer covers can easily double this cost. Figure 6-13 is an example that summarizes relative costs between capillary barrier cover (i.e., covers with capillary barrier effect (CCBE), desulphurization covers, and water covers for a given site. Desulphurization may be the most attractive alternative of the three options.
Partial Desulphurization and Water Cover (Bussiere and Wilson, 2006)

Data requirements for detailed design of prevention and mitigation strategies include detailed site characterization, such as topography and physical setting, geology, hydrogeology and hydrology, climate, materials availability and mine development sequence, and detailed characterization of source materials, including type, geochemistry, volume, and reactivity. Evaluation of alternatives is discussed further in Chapter 9.
6.9 Design and Construction Considerations
Design of prevention and mitigation measures will likely require some analytical or numerical modeling (or both analytical and numerical modeling) to predict both geochemical and physical performance (see Chapter 5). The time frame and scheduling of the implementation of control measures becomes important when PAG materials have a limited time to the onset of ARD production.
Construction must consider site location and transportation logistics, use and availability of local material types, quality control and quality assurance, including as-built inspection and reporting, and ongoing monitoring, maintenance, and reporting requirements.
Construction quality control programs are critical to the success of any prevention and mitigation measure.
6.10 Maintenance and Monitoring Considerations
Effective maintenance and monitoring programs must follow selection and implementation of any technical method for prevention or mitigation. Monitoring demonstrates achievement of objectives and maintenance ensures engineering integrity of the design. Monitoring is discussed in more detail in Chapter 8.
6.11 References
- Australian Centre for Geomechanics (ACG). (2006). Paste and Thickened Tailings – A Guide. Editors R.J., Jewell and A.B., Fourie. Nedlands, Western Australia, Australia, 2nd Edition.
- Acid Drainage Technology Initiative (ADTI). (1998). Handbook of Technologies for Avoidance and Remediation of Acid Mine Drainage. J.Skousen, A. Rose, G., Geidel, J., Foreman, R., Evans, W. Hellier, and members of the Avoidance and Remediation Working Group. National Land Reclamation Centre, West Virginia University, Morgantown, West Virginia.
- Aubertin, M. Industrial NSERC Ecole Polytechnique –UQAT Chair. (2005). (unpublished presentation).
- British Columbia Acid Mine Drainage Task Force (BC AMD). (1989). Draft Acid Rock Drainage Technical Guide .BiTech Publishers Ltd. Vancouver, BC. Canada.
- Bussière, B. (2007). Colloquium 2004: Hydrogeotechnical properties of hard rock tailings from metal mines and emerging geoenvironmental disposal practices. Canadian Geotechnical Journal. 44: 1019-1052.
- Bussiere, B. and Wilson, G.W. (2006). Cover Design Manual for Peru. CIDA - Percan project
- Claypool, G., J., Cassie, and R. Carreau. (2007). Reclamation of Frozen Sulphidic Tailings Area – Nanisivik Mine, Northern Canada. Proceedings of the 2nd International Seminar on Mine Closure. October 16-19, (2007). Santiago, Chile. Australian Centre for Geomechanics, Nedlands WA, Australia.
- Department of Water and Forestry, Republic of South Africa (DWAF) (2006). Best Practice Guideline – H3: Water Reuse and Reclamation. Pulles, Howard & de Lange Inc.
- DWAF (2007). Best Practice Guideline – H2: Pollution Prevention and Minimization of Impacts. ERMITE (Environmental Regulation of Mine Waters in the European Union) (2003).Technical and Managerial Guidelines D6. P.L. Younger and C. Wolkersdorfer Editors.
- Lang, B. (2007) http://www.cerm3.mining.ubc.ca/millenium.htm
- Mine Environment Neutral Drainage (MEND), (1993). Panel Wetland – A Case History of Partially Submerged Pyritic Uranium Tailings Underwater. Report 3.12.2.
- MEND, (Mine Environment Neutral Drainage Program) (1998). Blending and Layering Waste Rock to Delay, Mitigate or Prevent Acid Rock Drainage and Metal Leaching: A Case Study Review. Report 2.37.1. CANMET-MMSL.
- MEND, (1998). Design Guide for the Subaqueous Disposal of Reactive Tailings in Constructed Impoundments. Report 2.11.9.
- MEND, (2001). Prevention and Control. Volume 4. Manual 5.4.2d Gilles A. Tremblay and Charlene M. Hogan Editors. CANMET.
- MEND, (2004). Covers For Reactive Tailings Located in Permafrost Regions Review. Report 1.61.4. Natural Resources Canada. W.A. Price. CANMET.
- MEND, (2007). Macro-Scale Cover Design and Performance Monitoring Manual. Report 2.21.5. O’Kane Consultants Inc. Editors. Natural Resources Canada.
- Miller, G., and Van Zyl, D. (2008). Personal communication. See also http://www.unr.edu/passivation/
- Miller, S., Rusdinar, Y., Smart, R., Andrina, J., Richards, D. (2006). Design and Construction of Limestone Blended Waste Rock Dumps – Lessons Learned from a 10-Year Study at Grasberg. Proceedings of 7th International Conference on Acid Rock Drainage. March 26-30, (2006) St. Louis, MO.R.I. Barnhisel (ed.) American Society of Mining and Reclamation.Lexington KY, USA.
- Miller, S., Smart, R., Andrina, J., Neale, A., Richards, D. (2003). Evaluation of Limestone Covers and Blends for Long-Term Acid Rock Drainage Control at the Grasberg Mine, Papua Province, Indonesia. Proceedings of 6th International Conference on Acid Rock Drainage. July 12-18, 2003. Cairns, QLD, Australia. AusIMM. 133-141.
- Ouangrawa, M., Molson, J., Aubertin, M., Zagury, G., Bussière, B. (2006). The Effect of Water Table Elevation on Acid Mine Drainage from Reactive Tailings: A Laboratory and Numerical Modeling Study. Proceedings of 7th International Conference on Acid Rock Drainage. March 26-30, 2006 St. Louis, MO. R.I. Barnhisel (ed.) American Society of Mining and Reclamation. Lexington KY, USA.
- Pennsylvania Department of Environmental Protection (PaDEP) (1998). Coal Mine Drainage Prediction and Pollution Prevention in Pennsylvania. K.B.C. Brady, M.W. Smith, J. Schueck, Editors. Harrisburg, PA, USA. (Chapters 12 thru 17) posted at: http://www.dep.state.pa.us/dep/deputate/minres/Districts/CMDP/.
- Peel, M. C., and Finlayson, B. L. and McMahon, T. A. (2007). "Updated world map of the Köppen-Geiger climate classification". Hydrol. Earth Syst. Sci. 11: 1633-1644.
- Pulles, W. (2008) Personal Communication. DWAF Best Practice Guideline was in print during writing of this version. Golder Associates, Midrand, South Africa.
- Sjoberg-Dobchuk, B., Wilson, G.W., and Aubertin, M., (2003). Evaluation of a Single-Layer Desulfurised Tailings Cover, Sixth International Conference Acid Rock Drainage, Cairns, Australia, 14 – 17 July.
- Taylor, G., A. Spain, A. Nefiodovas, G. Timms, V. Kuznetsov, and J. Bennett. (2003). Determination of the Reasons for Deterioration of the Rum Jungle Waste Rock Cover. ACMER, Brisbane.
- Taylor, J.R., Guthrie, B., Murphy, N.C., and Waters, J. (2006). Alkalinity Producing Cover Materials for Providing Sustained Improvement in Water Quality from Waste Rock Piles. Proceedings of the 7th ICARD, March 26-30, St. Louis, USA.
- Northern Territory Minerals Council (Inc.) and the Mines and Petroleum Management Division of the Northern Territory Government (TEAM NT). (2004). TEAM NT: Technologies for Environmental Advancement of Mining in the Northern Territory: Toolkit, D.R. Jones and M. Fawcett, principal authors. Posted on the Northern Territory Minerals Council web page at http://ntminerals.org.au/VisionEdit/files/TEAMNT.pdf.
- United States Environmental Protection Agency (USEPA) (2003). EPA and Hardrock Mining: A Source Book for Industry in the Northwest and Alaska. Prepared by EPA Region 10 with the technical assistance of Science Applications International Corporation.
- Wickland, B.E., G.W. Wilson, D. Wijewickreme, B. Klein. (2006). Design and evaluation of mixtures of mine waste rock and tailings. Canadian Geotechnical Journal. 43: 928-945.
- Williams, D.J., Gowan M., Keefer P. (1995). Practical Co-Disposal Deposition. In: Proceedings of 7th Australian Coal Preparation Conference, Mudgee, New South Wales, Australia, pp. 371-383.
- Wilson, G.W. (2008a). Why are we still struggling with ARD. Sixth Australian Workshop on Acid and Metalliferous Drainage? Burnie, Tasmania. 15 – 18 April.
- Wilson, G.W. (2008b).Permission given for use in GARD Guide. ACMER. Brisbane.
- Wilson, G.W., D.J. Williams, and E.M. Rykaart, (2003). The Integrity of Cover Systems - An Update. Proceedings of 6th International Conference on Acid Rock Drainage, "Application of Sustainability of Technologies"; Cairns, Queensland, Australia. AusIMM: 445-452.
List of Tables
- Table 6-1: Forms of Codisposal
- Table 6-2: Benefits and Considerations of Codisposal
- Table 6-3: Benefits and Limitations of Alkaline Amendments
- Table 6-4: Considerations and Limitations of Soil Covers
- Table 6-5: Benefits and Disadvantages of Synthetic Covers
- Table 6-6: Some Considerations for Subaqueous Disposal
- Table 6-7: Summary of Prevention and Mitigative Measures and Climate Considerations
List of Figures
- Figure 6-1: Options and Effectiveness with Time (TEAM NT, 2004)
- Figure 6-2: Waste Rock Pile Structure and Processes (Wilson, 2008b)
- Figure 6-3: Adaptive Management Implementation by Phased Approach
- Figure 6-4: Methods for Prevention and Mitigation of ARD
- Figure 6-5: Coefficient of Diffusion versus Degree of Saturation for Saturated Porous Media
- Figure 6-6: Example Waste Rock Encapsulation Strategy
- Figure 6-7: Covers and Climate Types (from Holdridge et al., 1971)
- Figure 6-8: Sample Soil Covers Designs (MEND, 2001)
- Figure 6-9: Sample Configurations of Synthetics in Soil Covers
- Figure 6-10: Subaqueous Tailings Disposal
- Figure 6-11: Water Cover Processes
- Figure 6-12: Prevention and Mitigation Evaluation of Alternatives
- Figure 6-13: Comparative Costs for Barrier Cover (CCBE), Complete and Partial Desulphurization and Water Cover (Bussiere and Wilson, 2006)